
Electrical Stimulation Restores Ability to Walk in People With Paralysis
The findings are a first step towards developing targeted treatment and improving quality of life for people with spinal cord injuries.
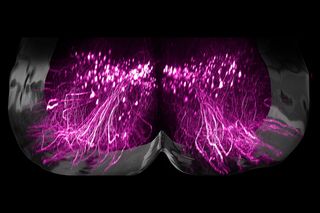
A mix of electrical stimulation and physical rehabilitation has restored the ability to walk in nine people who developed severe or complete paralysis due to spinal injuries, reported ScienceAlert. Now, scientists have identified the exact neurons responsible for allowing paralysed people to walk again – a finding that could potentially lead to more targeted treatment options for those with spinal cord injuries.
The team, in their study published in the journal Nature Medicine, said that nine participants had undergone EES and intensive therapy with the assistance of a robotic support system that aided their movement in different directions. After five months, all participants were able to take steps with the help of a walker, while four of them no longer needed the EES transmitter to be switched on to walk. “This sustained recovery suggests that the stimulation triggers remodelling of the spinal neurons to bring the locomotion network back on line,” noted an article in Nature.
These neurons are located in the lower region of the spinal cord. The brain sends signals through pathways that activate these neurons. In cases of spinal cord injuries, this communication is hampered. Even if the neurons responsible for walking are not damaged, the inability to receive signals from the brain due to an injury might lead to them becoming nonfunctional, resulting in permanent paralysis.
In 2018, the team of researchers had shown that when transmission of electrical pulses to the spinal cord through a surgically implanted neurotransmitter – a process known as epidural electrical stimulation (EES) – was combined with physical therapy, it could restore locomotive function. Participants not only regained voluntary control over their previously paralysed muscles, but were soon able to walk as well. However, the exact mechanism of how this worked was unclear at the time. To investigate this further, the researchers, who are part of the Swiss research group NeuroRestore, tested this technology in nine individuals as well as in mice.
The researchers also noticed a decrease in neural activity in the lower regions of the spinal cord in participants after treatment. They inferred that the electrical stimulation was probably activating only a subpopulation of neurons to initiate recovery. “When you think about it, it should not be a surprise… [B]ecause in the brain, when you learn a task, that’s exactly what you see – there are less and less neurons activated,” as one gets better at the task, neuroscientist Grégoire Courtine, who was part of the study, told Nature.
Related on The Swaddle:
New Findings Offer Hope for Recovery From Paralysis From Spinal Cord Injuries
When this treatment – right from injury to electrical stimulation to physical therapy – was modeled in mice, the researchers observed results consistent with those in humans. According to ScienceAlert, they also measured gene activity in spinal tissues of mice, identifying a single population of “previously unknown neurons” located in the lumbar spinal cord that seem to be important for recovery post injury.
This conclusion was drawn when the researchers noted that EES no longer allowed the mice to recover and regain their ability to walk when these neurons were silenced. Meanwhile, blocking the activity of these neurons in healthy mice did not affect their walking abilities, revealing that these neurons have a significant role to play specifically in recovery after spinal injuries.
A lot more remains to be investigated as this neural activity is only one part of a larger neural network in the brain and spinal cord that must also contribute to walking and recovery post injury, the researchers noted in their study.
While the neurons were identified in mice, Eiman Azim, a neuroscientist at the Salk Institute for Biological Studies, believes these neurons could also be responsible for producing these results in humans too. Azim told Nature that spinal architecture is similar across vertebrates, including in mice and humans. A more in-depth understanding of spinal cord neural activity could, then, help develop precise treatments, where neuroscientists could, one day, manipulate the activity of specific neurons using gene therapy.
EES is “one of the few [advances] that has shown remarkable changes in performance,” Arun Jayaraman, a clinician and scientist at the Shirley Ryan AbilityLab told Science. He added that these findings could potentially help doctors better target the correct nerves during therapy and reduce possible side-effects – such as an inability to empty the bladder fully – that may arise when the entire spinal cord is stimulated.
“The amount of hope that it gives to people with spinal-cord injury is incredible,” Marc Ruitenberg, a neurologist at the University of Queensland told Nature. Ruitenberg added that while restoring the ability to walk is an incredible achievement, there are other factors that can have a greater impact on people’s quality of life, such as loss of bladder control, sexual function and bowel control. “It would be really interesting to see whether those sorts of functions also can be improved with this technology,” he said.
However, a lot more research needs to be conducted before epidural electrical stimulation can be instituted as a treatment option for patients with spinal cord injuries.
Ananya Singh is a Senior Staff Writer at TheSwaddle. She has previously worked as a journalist, researcher and copy editor. Her work explores the intersection of environment, gender and health, with a focus on social and climate justice.
Related


NFTs Don’t Have Any Ethical Use Cases, According to New Research
