
People Receive Lab‑Grown Blood in World‑First Clinical Trial
If proven effective, lab-grown blood could potentially revolutionize treatment for people with blood disorders and rare blood types.
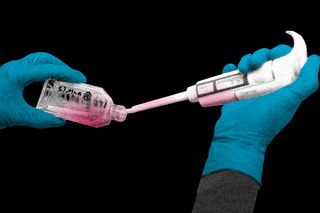
In a first-of-its-kind clinical trial, blood grown in the laboratory has been transfused into people. If this technology is proven effective, lab-grown blood could potentially revolutionize treatment for people with blood disorders and rare blood types. Researchers say this trial is a significant first step toward making lab-grown red blood cells available as a future clinical product.
“We hope our lab grown red blood cells will last longer than those that come from blood donors. If our trial, the first such in the world, is successful, it will mean that patients who currently require regular long-term blood transfusions will need fewer transfusions in future, helping transform their care,” said Cedric Ghevaert, the trial’s chief investigator from the University of Cambridge.
The red blood cells were grown from stem cells received from donors. Small amounts — one to two teaspoons — have been injected into two volunteers as part of the RESTORE clinical trial in the UK. The aim is to study the lifespan of the manufactured red blood cells in the body, to see how well they perform as compared to transfusions of standard red blood cells from the same donor.
The researchers are expecting the lab-grown cells to perform better and last longer, since they are fresher than the donated blood cells that contain cells of all ages. If successful, the trial could have positive implications for addressing blood transfusion needs.
If the manufactured cells last longer than the 120 days average lifespan of standard red blood cells, it could reduce the frequency of blood transfusions needed by patients. Transfusions that take place too often can lead to an overload of iron in the body, resulting in several complications.
Related on The Swaddle:
Researchers Identify a New, Rare Blood Type
Moreover, for those with rare blood types who require regular transfusions due to disorders such as sickle cell disease and thalassemia, this landmark development could address the severe shortage of donors as well. In India, a higher prevalence of blood disorders and complications during pregnancy means the country relies heavily on transfusions. The World Health Organization recommends that for every 1,000 people, there should be 10-20 donors to provide an adequate supply of blood. The ramifications of mismatched blood types, complex transfusions and rare blood types is compounded by a shortage of blood donors, posing a health risk for many patients.
Blood types are determined by the presence or absence of antigens on the surface of red blood cells. If transfused blood does not match that of the receiver, it could trigger alloimmunization – the generation of antibodies against a blood group antigen. This poses several complications. For instance, during pregnancy where the mother’s and fetus’s blood mixes through the placenta, it could cause an immune reaction that could result in the breakdown of red blood cells in the fetus or even the death of the unborn child.
Over the years, researchers have discovered blood group systems that go beyond the popularly-known ABO and rhesus (Rh) systems. Earlier this year, scientists found the rare Er blood group system. The rarest blood type known to us today is the Rhnull blood group – also known as “golden blood” – that completely lacks Rh antigens. Only 43 people in the world reportedly possess it.
Related on The Swaddle:
Anemia Is Often Seen as Iron Deficiency, But It Has Many Causes
While blood transfusions will continue to rely on donors for the foreseeable future, lab-grown red blood cells could prove beneficial for a small number of patients with complex transfusion needs, the researchers noted in a statement. The BBC, however, reported possible challenges that could arise when attempting to institute manufactured blood for clinical use, including the heavy costs of growing blood in the laboratory.
Harvested stem cells can also be quickly exhausted, limiting the amount of blood that can be grown. According to The Guardian, the stem cells are placed in a nutrient solution for 18-21 days to allow them to multiply. Around 24 liters of this solution are needed to produce a few spoonfuls of red blood cells.
The two people who have received lab-grown red blood cells are being closely monitored and seem to be doing well. These injected cells were tagged with a tracer that would allow researchers to detect them in blood samples taken from participants six months later. The next stage of the trial will involve a minimum of 10 volunteers who will receive small, randomized transfusions of both donated red blood cells and lab-grown blood, four months apart.
The researchers claimed that while further trials are needed before lab-grown blood can be clinically used, the study’s findings spell hope for many with complex transfusion needs. Professor Ashley Toye, an investigator in the trial, expressed excitement at the possibilities, saying, “This challenging and exciting trial is a huge stepping stone for manufacturing blood from stem cells. This is the first-time lab grown blood from an allogeneic donor has been transfused and we are excited to see how well the cells perform at the end of the clinical trial.”
Ananya Singh is a Senior Staff Writer at TheSwaddle. She has previously worked as a journalist, researcher and copy editor. Her work explores the intersection of environment, gender and health, with a focus on social and climate justice.
Related

Severe, Long‑Term Depression Can Reduce People’s Brain Volumes: Study
